As you are now more independent and in charge of your health care, you get to take the lead on making sure your bleeding disorders treatment is working for you. You may have questions about the different types of treatment options. This section has answers to common questions about factor replacement therapy. There are many factor replacement therapy products available. This section can help you develop your own set of questions to ask your health care provider to work together find the best treatment option.
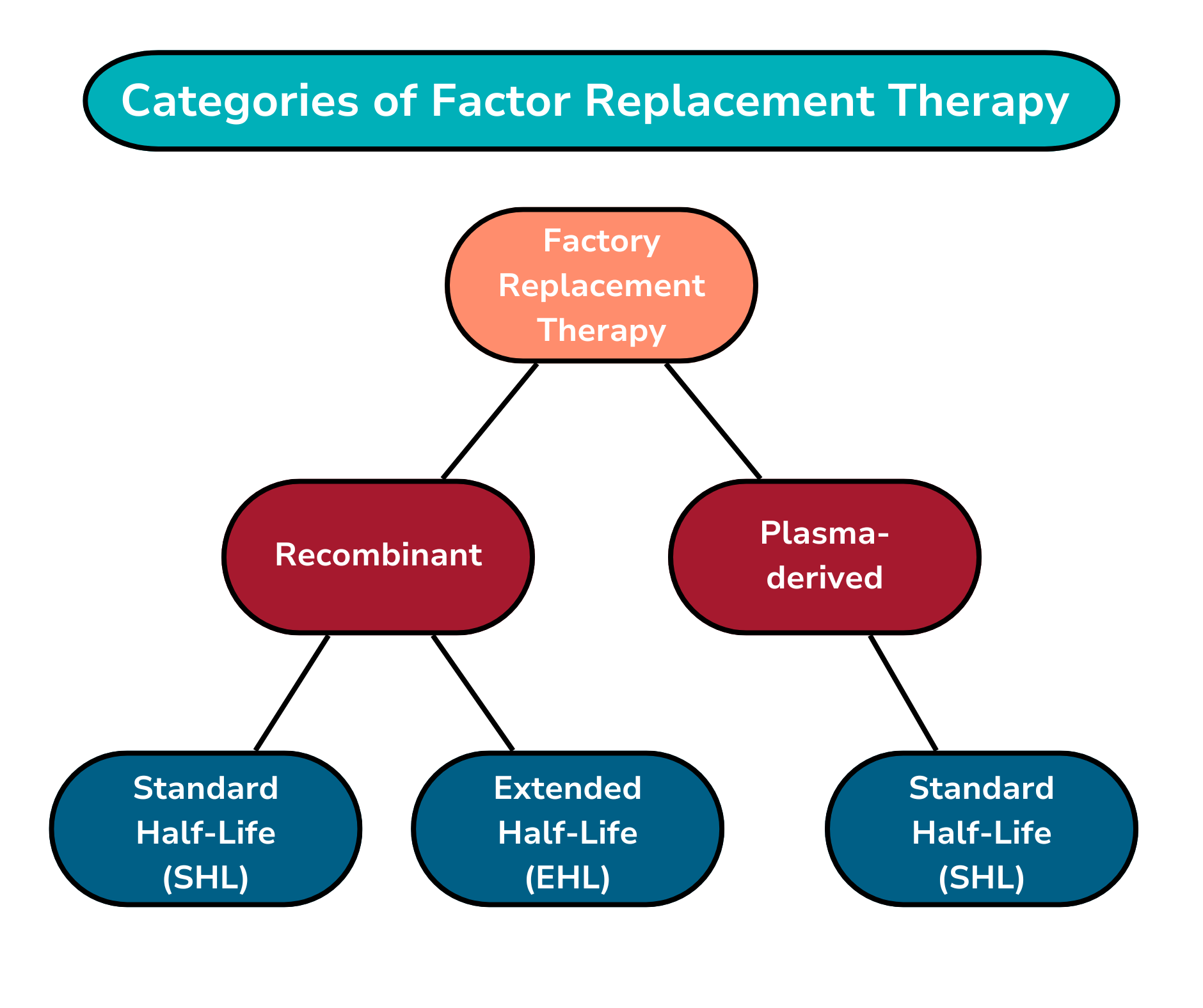
Clotting factor (called factor) is a dried powder form of the missing clotting factor. It is mixed with water to become a liquid before infusing it. Some clotting factor products, called plasma-derived factor, are made from donated human blood plasma. Others, called recombinant clotting factor, are made in a laboratory, and do not use human blood proteins.
When clotting factor is given, the level of factor in the blood rises. This rise lasts only for a certain number of hours based on the clotting factor’s half-life. Half-life is the amount of time it takes the body to use up half of the factor circulating in the body. Factor replacement therapies can have a shorter or longer half-lives depending on:
Which bleeding disorder you have.
How your individual body uses up (metabolizes) the clotting factor.
Whether you are using standard half-life (SHL) therapy or extended half-life (EHL) therapy.
Below is more information about standard and extended half-life therapies.
Standard half-life (SHL) therapies: SHL therapies are used to treat hemophilia A and B, some types of von Willebrand disease (VWD), and some ultra-rare factor deficiencies. The number of infusions a person may need can range from three times a week to every day, depending on the person.
Extended half-life (EHL) therapies: EHL therapies contain a molecule that has been altered. Which causes a delay in the breaking down the clotting factor in the body. EHL therapies result in higher levels of clotting factor in the body, which last for a longer time, resulting in less frequent infusions. How long the factor is effective in the body depends on the person. EHL therapies are used to treat hemophilia A and B. For more information on EHL therapies, please visit the Role of Prolonged Half-Life Clotting Factors in Hemophilia.
If you would like to learn more about these topics go to the NBDF’s Educational Programs website.
If some time has passed since you have treated and you still have pain, stiffness, or other signs of bleeding, you may need another dose of clotting factor. Sometimes another dose is recommended to be sure that a stable clot has formed, and the bleeding does not start again once the level of factor drops after treatment.
Your health care provider can provide exact instructions on when to repeat treatment.
Below is a chart that shows the categories of factor replacement therapy.
You will receive factor replacement therapy using a needle placed into your vein so that medicine goes through the needle into the vein (called infusion). If you want more information about how to do infusions, please go to Infusion Basics. This process takes time; it isn't like getting a quick shot. When you are first diagnosed and need immediate treatment, you will initially be treated at the Hemophilia Treatment Center (HTC), the physician's office, or the emergency department. Later, you (or your caregivers) will learn to infuse the factor at home (called home therapy). For more information about HTCs, please go to Hemophilia Treatment Centers.
Weight - As weight increases, dosages will tend to get larger.
The number of units in the bottle (called a vial) can vary depending on the product type.
The location of the bleeding site.
If it is a site of repeated bleeding (for example, if it is at a target joint).
If the bleeding could cause damage to the brain or other vital organs.
Some of the benefits of using factor replacement therapy include the following:
Replacing the missing factor needed for blood to clot.
Safe – recombinant products are recommended by NHF’s Medical and Scientific Advisory Council (MASAC) and when not available, plasma derived products are recommended as they have greatly improved in safety and monitoring.1
Brings factor levels into normal range.
Easy to measure factor levels with lab tests.
Years of experience with surgery and treating bleeds.
Both treats and prevents bleeds.
Some of the limitations with taking factor replacement therapy are as follows:
Treatment is given often (many times a week typically).
Must be given using an IV making it necessary to learn about ports and infusion. If you want to learn more about infusion, please visit Infusion Basics.
The potential formation of cells in your body that will attack the medication (called antibodies). This will cause the medication to be unable to work properly. This is also known as developing an inhibitor to the treatment. If you want more information about these antibodies, please go to Inhibitors.
Factor levels don’t stay within normal range very long. They go up and down (peaks and troughs).
If you are taking an EHLs, you can be in the 2-4% factor level longer than when you are taking a SHLs. This could put you at risk for bleeds during that timeframe. Make sure to discuss this with your health care provider.
Risk of blood borne diseases, such as hepatitis, from plasma derived products. This is because they contain human blood. Not all bleeding disorders have recombinant products available for treatment. Methods for screening blood and destroying blood-borne viruses have much improved. This makes medications created from blood products much safer to use. If you want more information about the safety of these products, please go to Blood Safety and Community Counts.
There is one factor replacement therapy, NovoSeven RT®, that has a Box Warning from the FDA. This warning is used to draw attention to the serious or life-threatening side effects or risks. You can find the warning on the box or in the product insert. It is important to speak with your health care provider about this box warning and what it would mean for you if you use this treatment product.
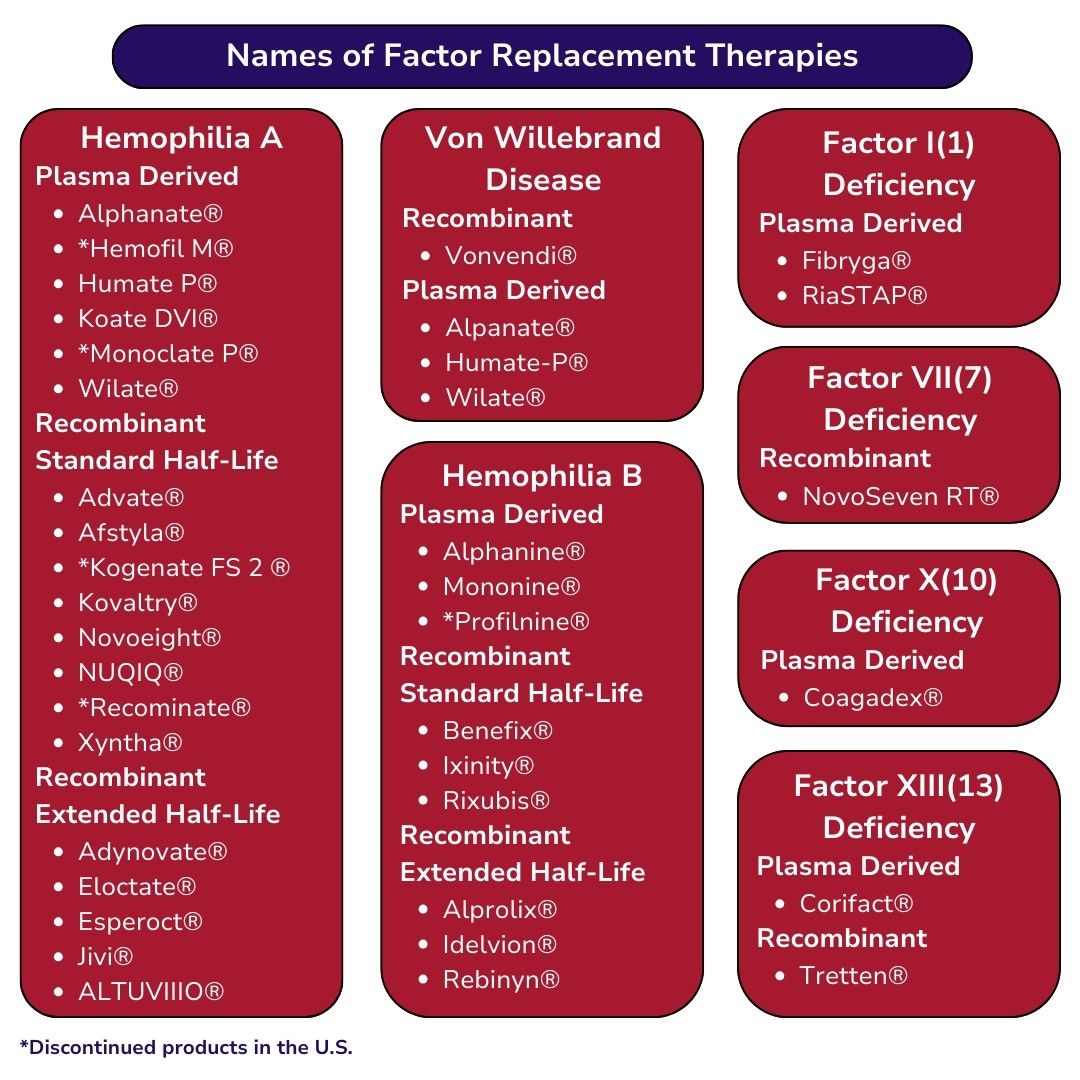
For information on all risks for each type and brand of factor replacement product, visit Products Licensed in the US | National Hemophilia Foundation
If you want more information about FDA-approved products for the treatment of bleeding disorders, please go to this list.” The link the word list to https://www.bleeding.org/healthcare-professionals/guidelines-on-care/products-licensed-in-the-us
NHF’s Medical and Scientific Advisory Council. (2022). MASAC Recommendations Concerning Products Licensed for the Treatment of Hemophilia and Other Bleeding Disorders (MASAC 272). National Hemophilia Foundation. https://www.bleeding.org/healthcare-professionals/guidelines-on-care/masac-documents/masac-document-290-masac-recommendations-concerning-products-licensed-for-the-treatment-of-hemophilia-and-selected-disorders-of-the-coagulation-system
NHF’s Medical and Scientific Advisory Council. (2016). Recommendations Regarding Doses of Clotting Factor Concentrate in the Home (MASAC 242). National Hemophilia Foundation. https://www.bleeding.org/healthcare-professionals/guidelines-on-care/masac-documents/masac-document-242-recommendations-regarding-doses-of-clotting-factor-concentrate-in-the-home